|
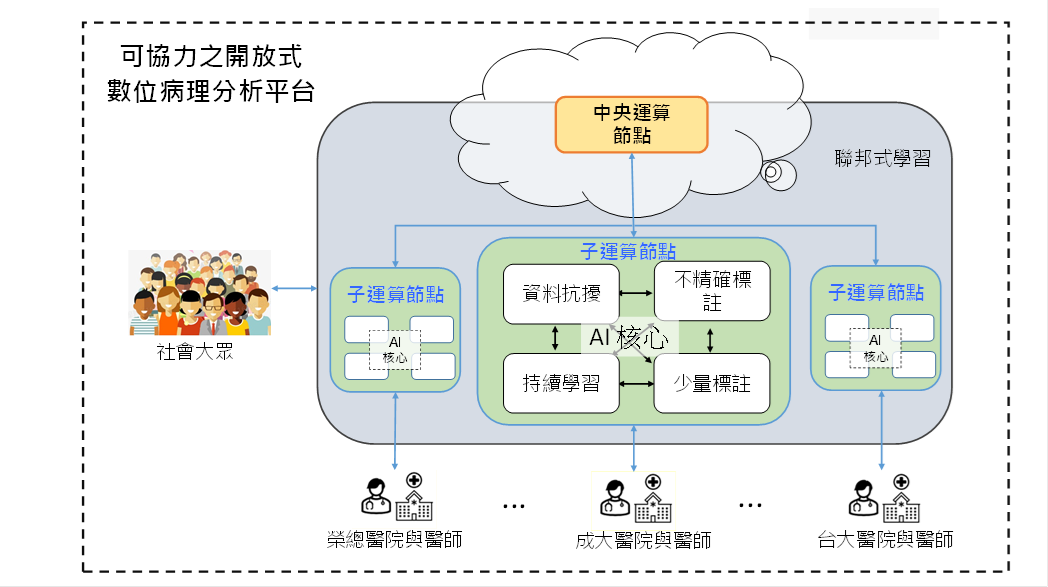
人工智慧(AI,artificial intelligence)數位病理分析是未來醫療的發展趨勢,可提供醫師客觀的病理分析結果並協助計算難以人為估計的量化病理指標,輔助醫師進行診斷,有助於提升病理診斷的效率與精確性。 |
Artificial intelligence (AI) digital pathology analysis plays a promising role in healthcare system. It provides physicians more objective analysis of the results, and helps them to calculate pathology indicators quantitatively, thus improving the efficiency and accuracy of pathology diagnosis. |
 |
|
|
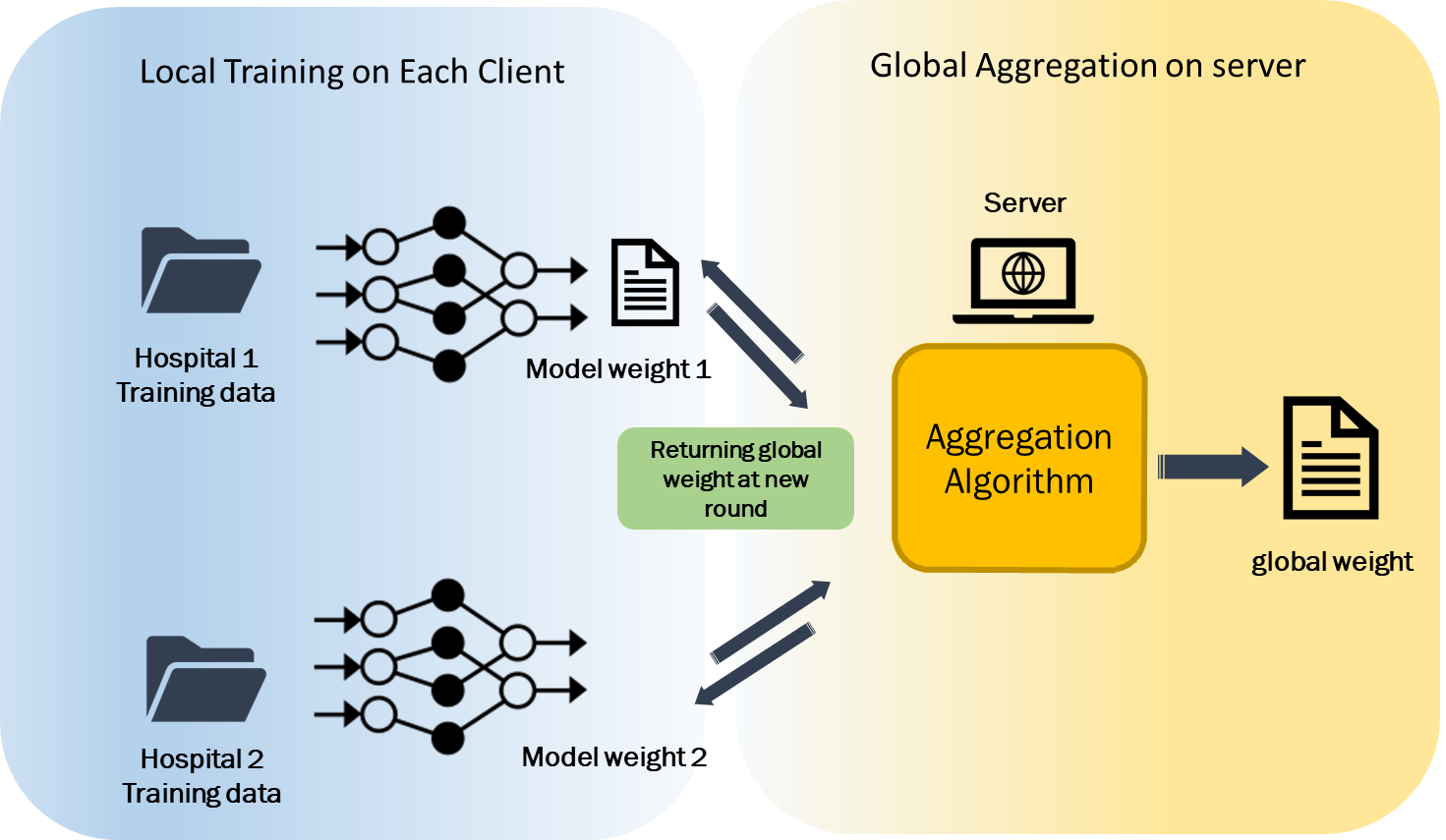
結合來自多間醫院的數位病理影像,可以大量提供發展數位病理AI分析模型所需的病理標註資料。然而為了保障病人隱私,醫療相關資料不適合與其他醫院交流,同時各間醫院病理資料的分布也不相同。我們以聯邦式學習為基礎,正在發展可有效率因應異質資料分布不均的協同式訓練平台,讓多間醫院無需交換資料也能共同發展數位病理AI分析模型。 |
Collecting data from different hospitals provides a large amount of data for developing pathology AI analysis models. However, data privacy is a critical issue. The data diversity from different hospitals also poses a great challenge to the model training. We are developing a novel federated learning based training platform to efficiently and effectively train AI models with heterogeneous data from different hospitals without exchanging the data. |
 |
|
|
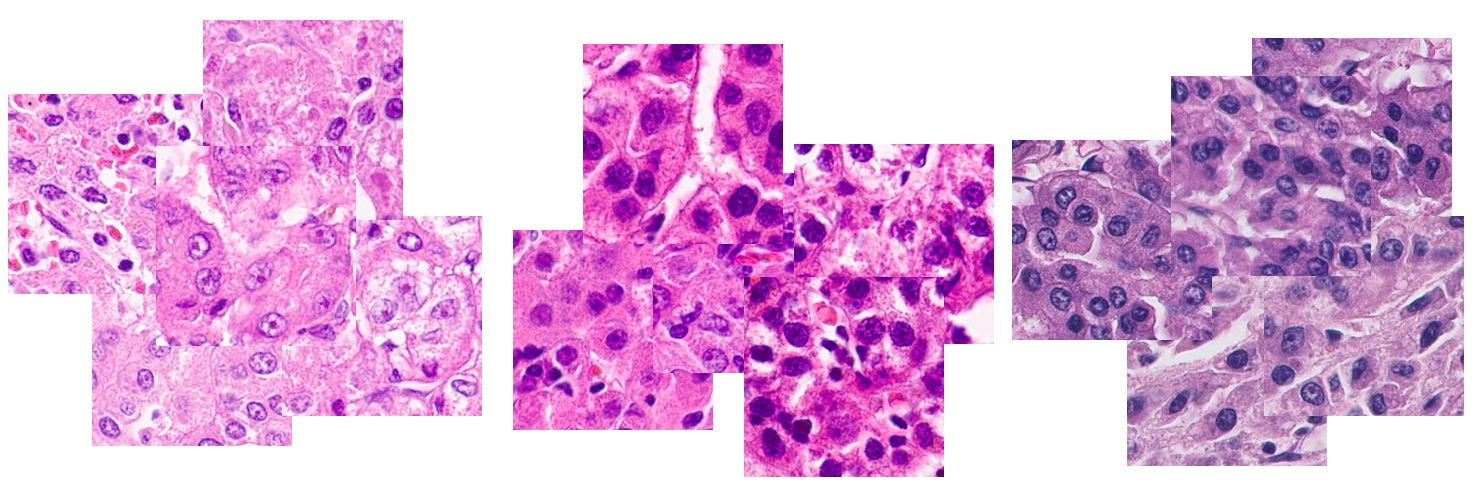
不同廠牌數位病理切片掃描器影像間往往會有極大的外觀與顏色差異,造成數位病理AI分析模型訓練的困難,也會嚴重影響AI模型的準確率。我們正在發展可有效保持AI模型準確率的影像變換,以及更加通用化,可適應多樣變化的AI模型。 |
The colors and appearance styles of the WSIs scanned by different scanners are often extremely diverse. This seriously degrade the performance of the pathology AI models. We are developing the cross-scanner transformation that can effectively retain the performance of the AI models. We also aim to build more generalized AI models that are robust to the cross-scanner variation. |
 |
|
|
全世界每年大約有 8百多萬人死於癌症,其中有 78萬死於肝癌。 而原發性肝癌占了全部肝癌的 90% 以上。透過外科手術以及術後治療,早期肝癌五年存活率可以提升至 50% 以上。 |
About 8 million people die of cancer worldwide each year, including 780,000 from liver cancer. Primary liver cancer accounts for more than 90% of all liver cancers. With surgery and post-operative treatment, the five-year survival rate for early-stage liver cancer can be increased to over 50%. |
 |
|
|
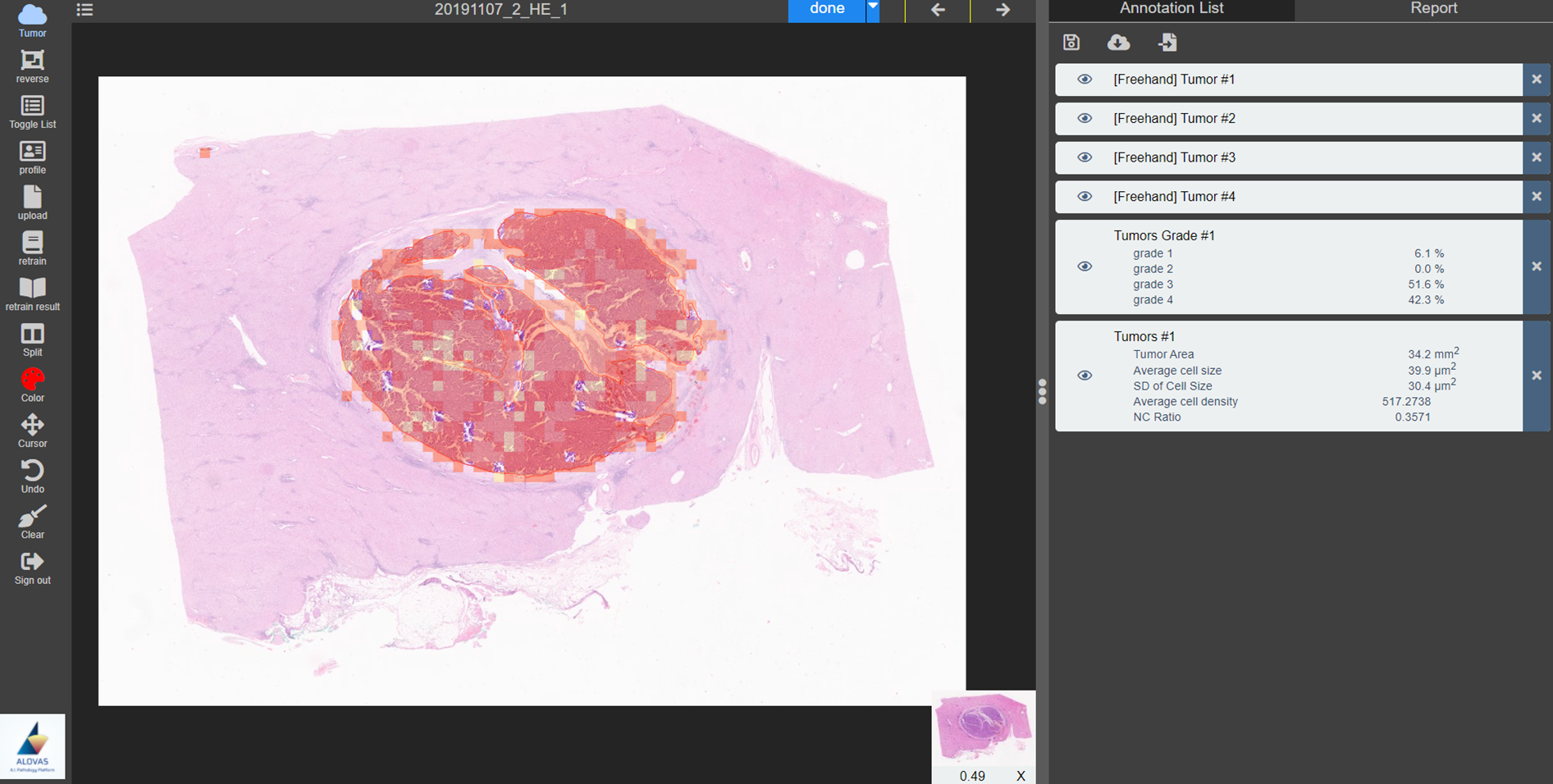
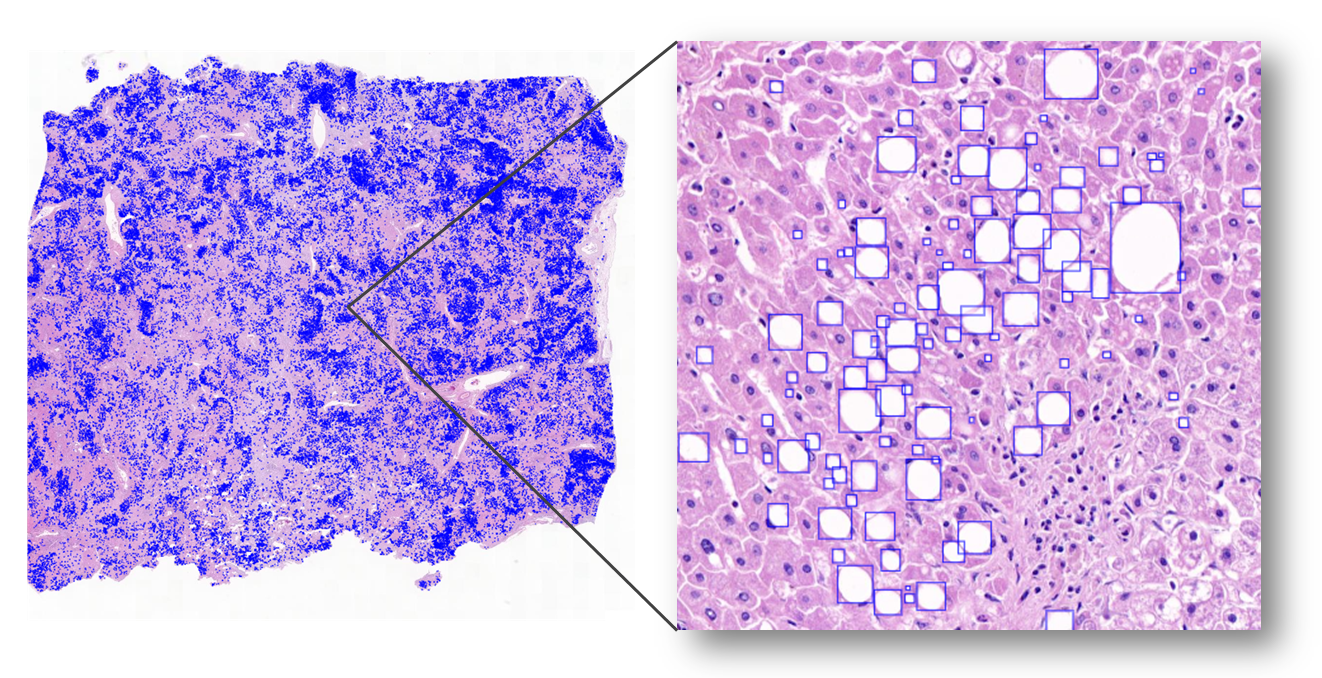
非酒精性脂肪肝(NAFLD)是一種極為常見的疾病,全球的患病率為 25.24% ,並具有潛在的嚴重影響可能會導致肝硬化,並與多種肝炎高度相關,隨著病情的發展可能惡化為肝癌,甚至導致肝功能喪失。我們提出結合油滴內部區域與邊緣區域的影像分割模型以更精準地偵測油滴區域,提供醫生進行脂肪變性分級的診斷。我們發展的模型正與成大醫院準備進行臨床驗證。 |
Non-alcoholic fatty liver disease (NAFLD) is an extremely common disease with a global prevalence of 25.24% and potentially serious effects that can lead to cirrhosis and is highly associated with many forms of hepatitis, which can progress to liver cancer and even loss of liver function. We have developed a novel segmentation model based on the segmentations of the internal and boundary regions of the droplets. The proposed model is under the preparation of clinical validation in National Cheng Kung University Hospital. |
 |
|
